Key research themes
1. How do small heat shock proteins (sHSPs) structure and oligomerization dynamics influence their chaperone function in protein folding and stress response?
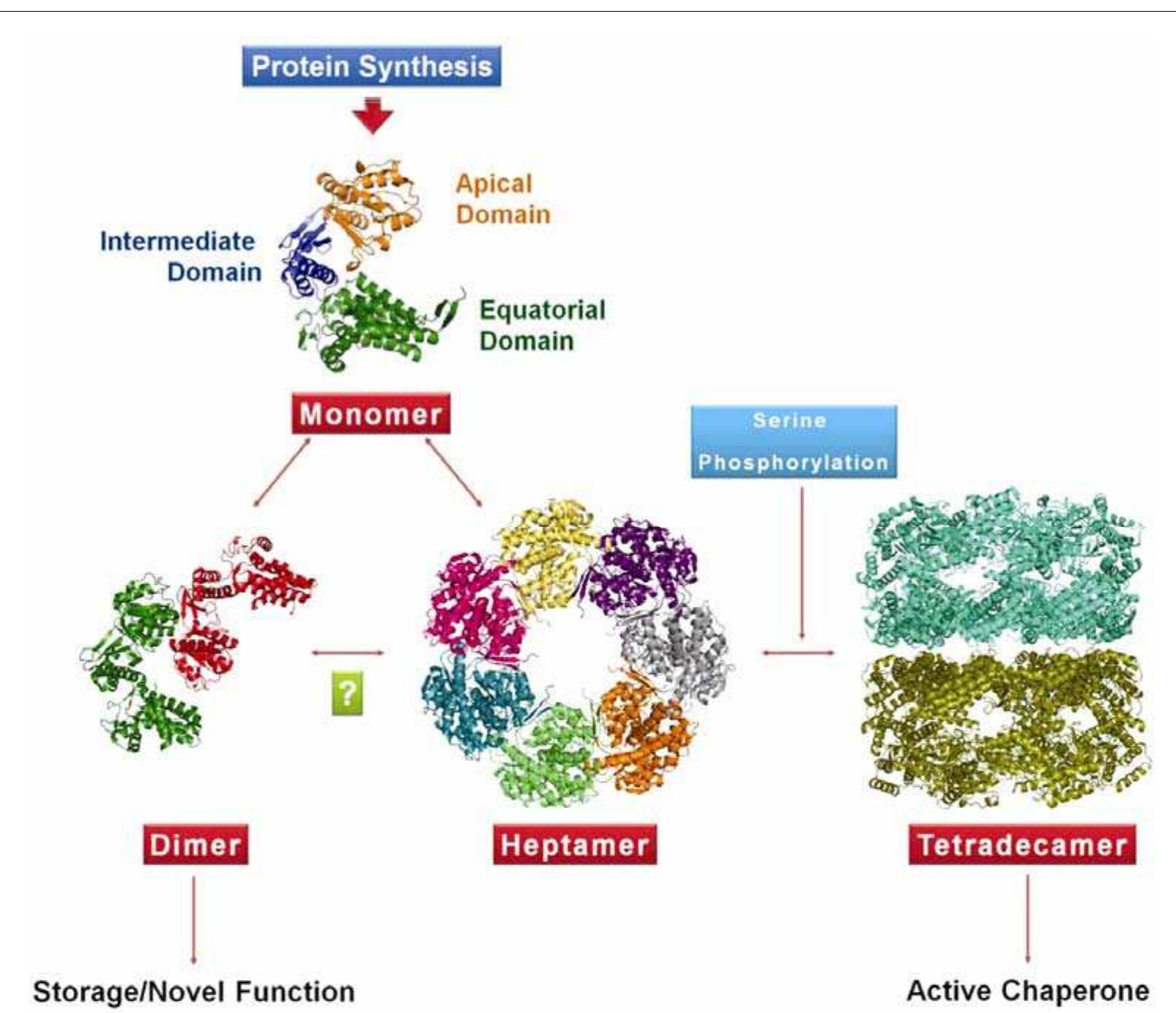
This research area investigates the molecular architecture of sHSPs, their oligomeric assemblies, dynamic subunit exchanges, and how these structural features underlie their ability to prevent protein misfolding and aggregation under stress. Understanding their diverse oligomeric states and the role of conserved and variable domains is crucial for linking chaperone activity with physiological functions and pathologies.
2. What are the mechanistic roles of Hsp70 family chaperones in protein folding pathways and cytoprotection?
This theme centers on understanding how Hsp70 chaperones interact with client proteins to prevent aggregation, facilitate folding and maintain proteostasis. It includes structural studies of Hsp70, its conformational states, binding dynamics, and influence on folding energy landscapes. Hsp70’s role as an ATP-dependent foldase and its cooperation with cochaperones is key to cellular stress responses and protein homeostasis.
3. How do heat shock proteins modulate immune responses and contribute to disease-related proteostasis and cytoprotection?
Research under this theme explores the extracellular and intracellular roles of HSPs, especially sHSPs and Hsp70s, in immune system modulation, inflammation, and diseases such as cancer, neurodegeneration, and diabetes. It emphasizes their dual pro- and anti-inflammatory functions, their interactions with immune receptors, and their role as danger signals or immunomodulants. Understanding these mechanisms has implications for developing therapeutic interventions targeting HSPs.