Figure 4 – uploaded by Santosh C M Kumar
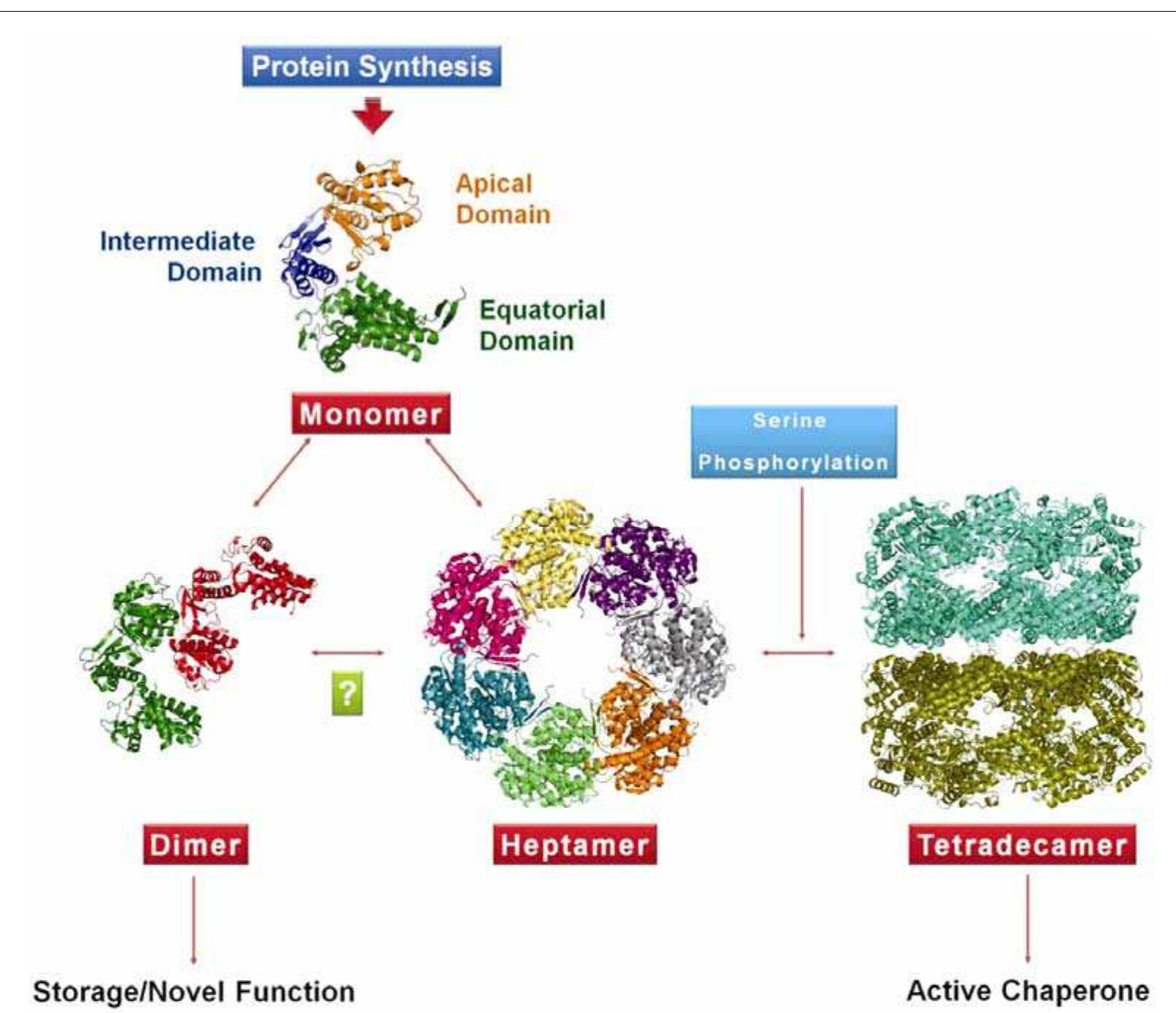
Figure 4 Model for the regulation of oligomerization in Mycobacterium tuberculosis GroEL1 mediated by phosphorylation. Upon synthesis, M. tuberculosis GroEL1 monomer might follow two pathways of oligomeriza tion. The monomers either assemble into a dimer or a heptamer. The dimer might act as a storage form or migh be involved in nucleoid formation. Function of the heptameric form is unknown. The signal for the probable con version of the dimeric form into heptameric form is yet to be discerned (shown with a question mark). However the signal for conversion of single-ring heptamer to double-ring tetradecamer is mediated by phosphorylation o1 serine residue(s). The tetradecameric GroEL is supposed to be the active form of the chaperonin.


