Synthesis and Characterization of Y2O3/SiO2 Composites
2004, Zeitschrift für Naturforschung A
Abstract
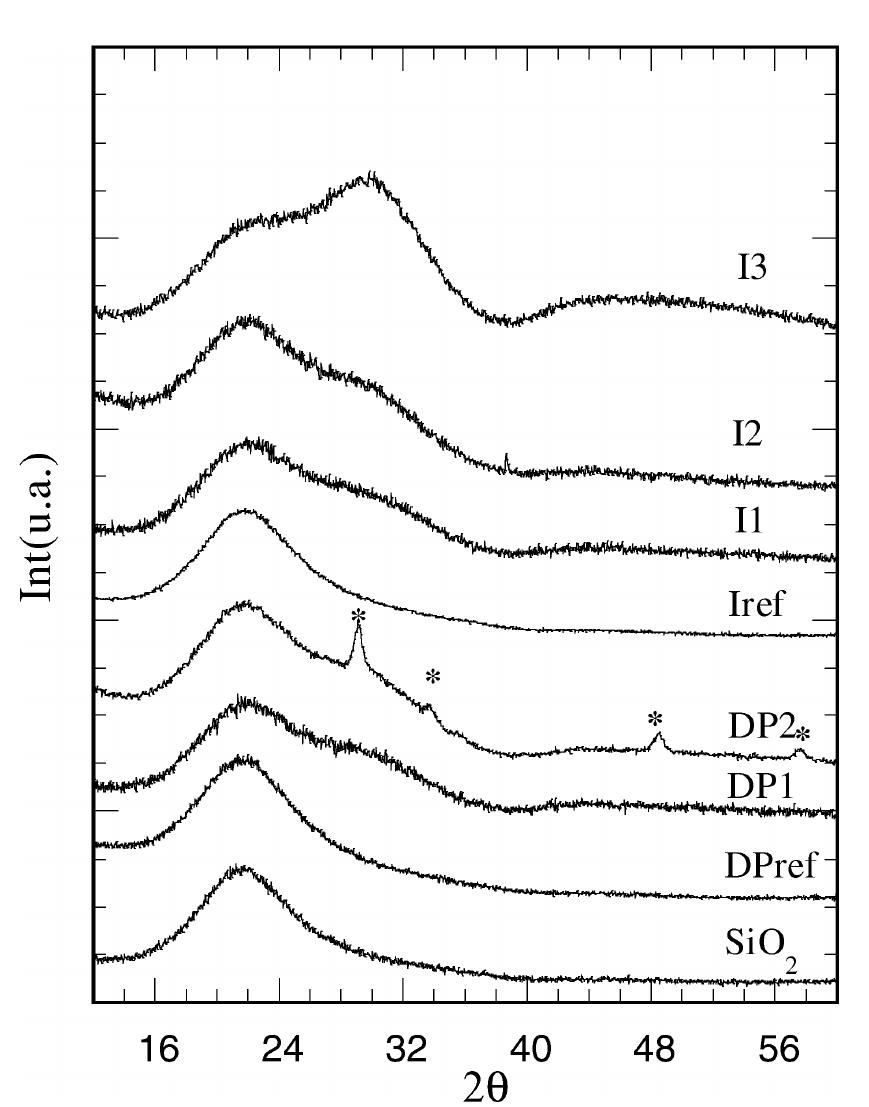
Impregnation and deposition-precipitation syntheses have been used to obtain Y 2 O 3 /SiO 2 samples. In the deposition method, the urea causes the precipitation of yttrium hydroxide, which leads to the formation of yttria nanocrystalline particles in the final composites. Delaying the silica addition up to the visible muddying of the solution, a relevant formation of yttria nanocrystalline particles with average size of about 12 nm is produced. The composites obtained through the impregnation method are amorphous and contain different amounts of yttrium depending on the kind of solvent, the highest concentration being reached using ethanol. In all the samples important interactions at the molecular level among yttrium and silica are revealed, but less important in composites obtained with the deposition precipitation method.
References (19)
- A. M. Lejeus and R. Collongues, Current Topics in Material Science. In: Kaldis E. editor, North-Holland Publ. Company, Amsterdam, Vol. 4, p. 481 (1980).
- T. B. Troczynski and P. S. Nicholson, J. Amer. Ceram. Soc. 72, 1488 (1989).
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, Berlin 1994.
- T. Ye, Z. Guiwen, Z. Weiping, and X. Shangda, Mater. Res. Bull. 3, 501 (1997).
- B. M. Tissue, Chem. Mater. 10, 2837 (1998).
- E. T. Golburt, B. Kulkarni, R. N. Bhargava, J. Taylor, and M. Libera, J. Lumin, 72, 190 (1997).
- Q. Li, L. Gao, and D. Yan, Chem. Mater. 11, 533 (1999).
- R. Schmechel, M. Kenedy, H. Von Seggem, H. Win- kler, M. Kolbe, R. A. Fischer, Li Xaomao, A. Benker, and M. H. Winterer, J Appl.Phys. 89, 1679 (2001).
- C. Cannas, M. Casu, A. Musinu, G. Piccaluga, A. Speghini, and M. Bettinelli, J. Non Cryst. Solids 193, 306 (2002).
- C. Cannas, M. Casu, A. Lai, A. Musinu, and G. Pic- caluga, Phys. Chem., Chem. Phys. 4, 2286 (2002).
- Y. D. Jiang, Z. L. Wang, F. Zhang, H. G. Paris, and C. J. Summers, J. Mater. Res. 13, 2950 (1999).
- Powder Diffraction File Card N • 43-1036 International Center for Diffraction Data, 1998, Swarthmore, PA USA.
- E. Lippmaa, M. Magi, A. Samoson, G. Enghelhardt, and A. R. Grimmer, J. Amer. Chem. Soc. 102, 4889 (1980).
- A. Bertoluzza, C. Fagnano, M. A. Morelli, V. Gottardi, and M. Guglielmi, J. Non-Cryst. Solids 48, 117 (1982).
- P. Hofmann and E. Knozinger, Surf. Sci. 188, 181 (1987).
- D. L. Wood and E. M. Rabinovich, Appl. Spectrosc. 43, 263 (1989).
- J. J. Chambers and G. N. Parsons, J. Appl. Phys. 90, 918 (2001).
- Powder Diffraction File Card N • 24-1422 International Center for Diffraction Data, 1998, Swarthmore, PA USA.
- C. Cannas, M. Casu, M. Mainas, A. Musinu, G. Pic- caluga, S. Polizzi, A. Speghini, and M. Bettinelli, J. Mater. Chem. 13, 1 (2003). View publication stats View publication stats
 Anna Musinu
Anna Musinu