Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure
- PMID: 17187081
- PMCID: PMC1705821
- DOI: 10.1172/JCI25483
Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure
Abstract
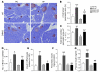
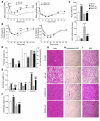
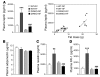
The incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) control glucose homeostasis through well-defined actions on the islet beta cell via stimulation of insulin secretion and preservation and expansion of beta cell mass. We examined the importance of endogenous incretin receptors for control of glucose homeostasis through analysis of Glp1r(-/-), Gipr(-/-), and double incretin receptor knockout (DIRKO) mice fed a high-fat (HF) diet. DIRKO mice failed to upregulate levels of plasma insulin, pancreatic insulin mRNA transcripts, and insulin content following several months of HF feeding. Both single incretin receptor knockout and DIRKO mice exhibited resistance to diet-induced obesity, preservation of insulin sensitivity, and increased energy expenditure associated with increased locomotor activity. Moreover, plasma levels of plasminogen activator inhibitor-1 and resistin failed to increase significantly in DIRKO mice after HF feeding, and the GIP receptor agonist [D-Ala(2)]GIP, but not the GLP-1 receptor agonist exendin-4, increased the levels of plasma resistin in studies of both acute and chronic administration. These findings extend our understanding of how endogenous incretin circuits regulate glucose homeostasis independent of the beta cell via control of adipokine secretion and energy expenditure.
Figures


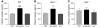
References
-
- Badman M.K., Flier J.S. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. - PubMed
-
- Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. - PubMed
-
- Mayo K.E., et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 2003;55:167–194. - PubMed
-
- Drucker D.J. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 2003;17:161–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
